Advanced breast cancer patients treated with oral vinorelbine: a prospective and retrospective, observational study – VINOREAL
Advanced breast cancer patients treated with oral vinorelbine: a prospective and retrospective, observational study – VINOREAL
Advanced breast cancer patients treated with oral vinorelbine: a prospective and retrospective, observational study – VINOREAL
How is the study conducted?
The following diagram summarizes how the study is designed :
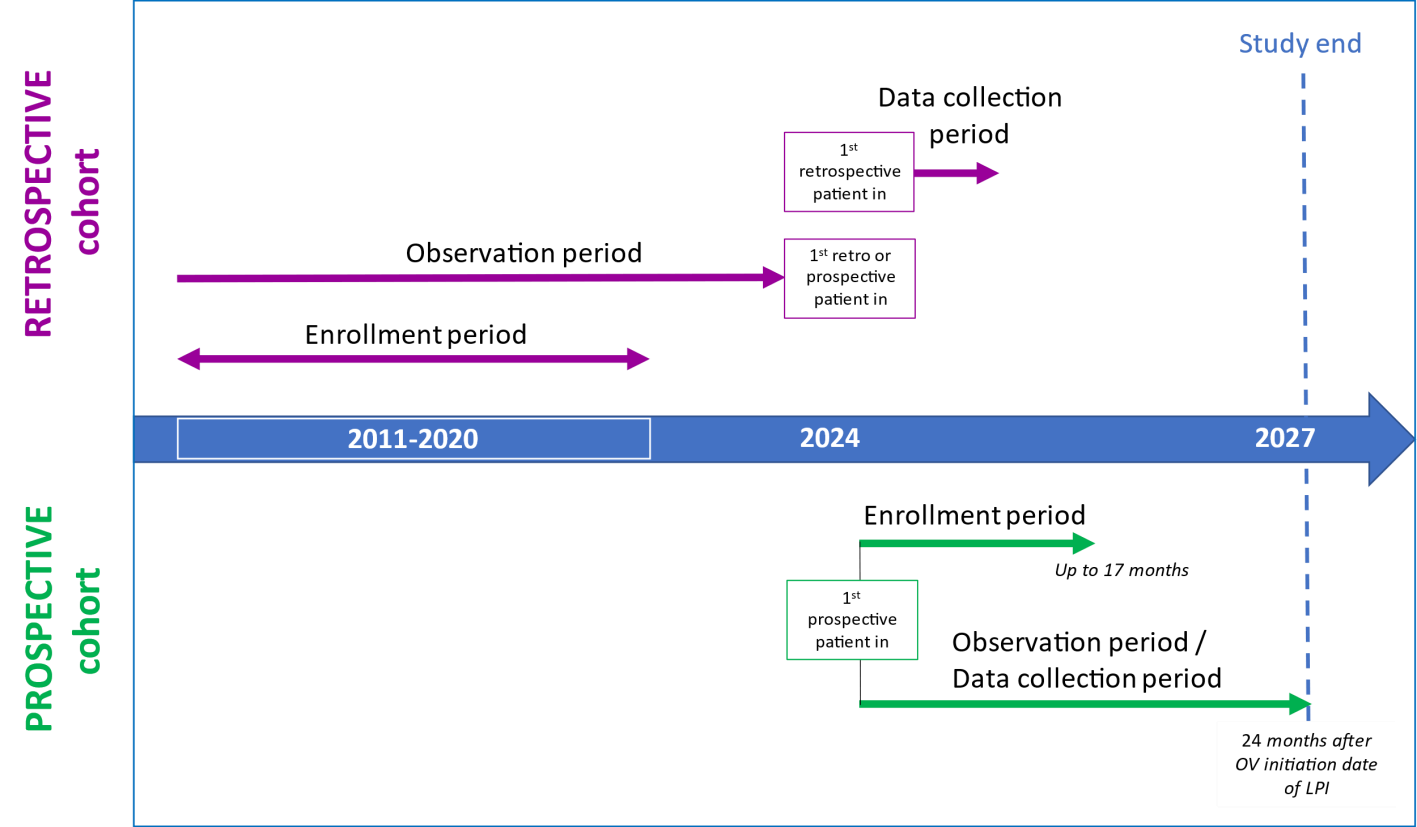
The enrollment period is the period during which patients will be included in the study based on the defined inclusion criteria.
- for the retrospective cohort: patients are included in the group based on their records. These patients started OV treatment for ABC at any time from 2011 to 2020 in this group. We will look back at their data.
-
for the prospective cohort: upcoming patients to be enrolled are patients who begin OV treatment when they join the study or after, and who can provide information over time about their quality of life and fill out surveys about their treatment preferences and how their illness affects their work and daily activities. They will be included in this group and will be followed up moving forward until two (2) years of treatment.
Who can take part in the trial ?
Patients can join the study if they meet all these requirements:
- Women of 18 years or older (19 years and older in Algeria according to local regulations) when they start taking the oral vinorelbine treatment
- Women diagnosed with an advanced form of breast cancer that can be treated with chemotherapy
- Women who started treatment with oral vinorelbine in accordance with its approved medical use for advanced breast cancer between January 2011 and December 2020 for the retrospective group and women who start or will be starting this treatment when they join the study for the prospective group.
- Women who are prospective willing and able to fill out surveys about their quality of life, their treatment preferences, and how their illness affects their work and daily life for the prospective group.
-
Women who have given their permission by signing a consent form, or if they can't, their family or legal representative has agreed to let their health information be collected and looked at, following what is allowed in their home country.
What are the objectives of the trial and how are they evaluated ?
The main (primary) objective is to explain how well a treatment based on OV works by looking at how long patients with ABC can live without their disease getting worse, after two years of being treated. This will be looked at in a group of patients who received OV treatment in the past (between 2011 and 2020) (retrospective part with the related retrospective cohort) as well as in a group of patients being treated now and, in the future, (prospective part with the related prospective cohort).
The secondary objectives are:
- To describe how effective a treatment with OV is at helping patients with ABC live longer, measured two years after starting the treatment. This will be looked at in a group of patients from the past (retrospective) and in a group of patients that are currently being treated or will be treated in the future (prospective).
- To describe how effective OV treatment is at helping patients with ABC live without their disease getting worse and at helping them live longer, specifically looking at a group of patients who received OV treatment in the past.
- To describe the demographics, clinical details, health conditions, and other illnesses of patients with ABC at the time of OV treatment initiation, and to see if these details are linked to certain characteristics of the cancer cells. This will be done by looking at patients from both retrospective and prospective groups.
- To explain what side effects patients with ABC might experience when treated with OV and how often these side effects happen, in both retrospective and prospective groups..
- To look at the real life treatment approaches for patients with ABC who are receiving OV, including what breast cancer treatments they had before, the dose of OV they received and length of OV treatment, any breaks or stops in OV treatment and the reasons for these breaks/stops, and any other treatments they are taking at the same time as OV, in both retrospective and prospective groups.
- To look at how patients with ABC treated with OV, access healthcare and their treatment journey, including hospital stays (including stays related to side effects from OV treatment), radiation therapy, and surgeries, in both retrospective and prospective groups.
- To evaluate how the quality-of-life changes over time for patients with ABC who are currently receiving OV treatment by using a survey (that is to say the Functional Assessment of Cancer Therapy questionnaire for breast cancer patients: FACT-B) that measures the health status of breast cancer patients.
- To find out what current patients with ABC being treated with OV prefer in terms of how they receive their OV treatment by asking them to fill out a survey (Patient Preference Questionnaire- PPQ) about their treatment preferences.
- To study how cancer affects the work and daily life of current patients with ABC who are treated with OV by using a survey (Work Productivity and Activity Impairment Questionnaire) that asks about work and activity levels.
