A trial to test if the combination of encorafenib, binimetinib and cetuximab is safe and effective in participants with BRAFV600E-mutant metastatic colorectal cancer.
A trial to test if the combination of encorafenib, binimetinib and cetuximab is safe and effective in participants with BRAFV600E-mutant metastatic colorectal cancer.

Phase 2, open-label, single arm, multicenter study of Encorafenib, Binimetinib plus Cetuximab in subjects with previously untreated BRAFV600E -mutant metastatic colorectal cancer
How is the study conducted?
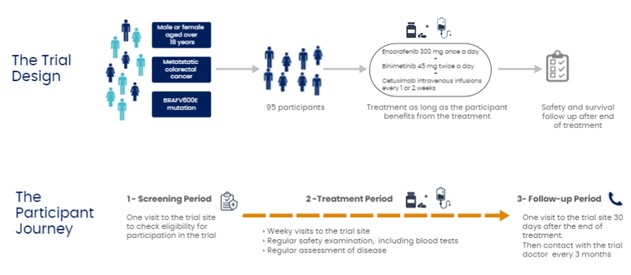
This is an open-label Phase II trial with 95 adult participants.
The trial consists of 3 different periods:
• A screening period during which participants attend a visit at the trial site to check if they meet all the criteria required for participation in the trial.
• A treatment period during which participants receive treatment with encorafenib, binimetinib and cetuximab for as long as they benefit from the treatment. That means as long as the disease does not worsen (disease progression) and the treatment is tolerated. During this period, the doctors perform physical examinations, blood tests and imaging tests to monitor the size of the tumour. They also regularly check participants' health and take note of any side effects.
• A follow-up period that begins when participants stop the treatment. This period consists of monitoring the participants’ health over time.
The picture below summarizes the information presented above:
Who can take part in the trial ?
To take part in the trial, participants must fulfill several conditions including the following:
• Participants aged 18 years or older with colorectal cancer that has spread to other places of the body (metastatic) and has a specific genetic mutation in BRAF gene (BRAFV600E).
• Participants have not yet received any therapy for their disease.
What are the objectives of the trial and how are they evaluated ?
The main objective of the trial is:
• To find out if the combination of encorafenib, binimetinib and cetuximab is effective in this type of colorectal cancer. This is assessed by evaluating the proportion of participants for whom the tumors shrink or completely disappear after treatment (overall response rate).
In addition, the trial will allow:
• To evaluate the time period the tumor continues to respond to treatment (duration of response).
• To evaluate the time period between the treatment start and the disease worsens (progression-free survival).
• To evaluate the time period between the treatment start and participant’s death (overall survival).
• To evaluate if the combination of encorafenib, binimetinib and cetuximab is safe by assessing the number, frequency and type of side effects.
What is the study Medication ?
Participants receive the following treatments:
• Encorafenib (BRAFTOVI®): 4 capusles to be taken once a day in the morning. The total daily dose of encorafenib is 300 mg.
• Binimetinib (MEKTOVI®): 3 tablets twice a day, in the morning and in the evening. The total daily dose of binimetinib is 90 mg.
• Cetuximab (ERBITUX®): intravenous infusions performed at the investigational sites every 1 or 2 weeks.
Duration of administration: as long as the participant benefits from the treatment. That means as long as the disease does not worsen (disease progression) and the treatment is tolerated.
