A trial to test an antibody as an experimental treatment for Patients with Advanced or Metastatic Solid Tumors (VISTA)
A trial to test an antibody as an experimental treatment for Patients with Advanced or Metastatic Solid Tumors (VISTA)
Phase I dose escalation and dose expansion, international, multicenter study of W0180 as single agent and in combination with pembrolizumab (anti-PD-1) in adult participants with locally advanced or metastatic solid tumors.
How is the study conducted?
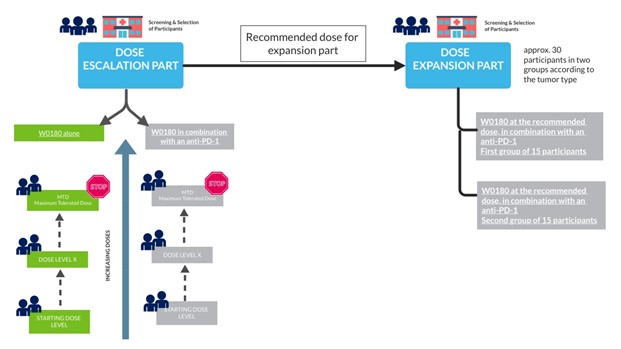
The Dose Escalation Part will include up to 39 participants either administered with increasing doses of W0180 alone or in combination with pembrolizumab.
The Expansion Part will include up to 30 participants with specific tumor types, at the dose determined following the dose escalation part.
The schema summarizes the study design:
Who can take part in the trial ?
To be part of the trial, participants must fulfil several conditions, including the following:
• Adult participants with locally advanced or metastatic solid tumors and following specificities according to trial part:
• For the dose escalation in combination with pembrolizumab and the dose expansion only: Participants whose diseases are not responsive to a certain class of immunotherapies
• For the expansion part only: Participants with tumor types expressing VISTA receptor
• Participants unresponsive to standard treatment or for whom no standard treatment is available or appropriate
What are the objectives of the trial and how are they evaluated ?
The main objective of the trial is to determine a best-tolerated dose that would be recommended for clinical development of W0180.
This relies firstly on the identification of the Maximum Tolerated Dose (MTD) that is to say the highest dose of W0180 (alone and in combination with pembrolizumab) that can be administered without exposing participant to an unacceptable health risk. The MTD is determined by testing increasing doses on different groups of participants (dose level) until the highest dose with acceptable side effects is found. Based on MTD evaluation and all othersinformation available at the end of the escalation part, a dose of W0180 will be recommended for the expansion part (RDE).
In addition, the trial will allow:
- To describe the safety of W0180 (alone and in combination with pembrolizumab) by evaluating the number and type of side effects
- To describe how the treatment enters the body, circulates in the bloodstream and is finally eliminated.
- To provide preliminary data on activity of W0180 (alone and in combination with pembrolizumab) against cancer tumors
What is the study Medication ?
W0180 is a therapeutic antibody.
An antibody is a molecule pertaining to the immune system that can specifically recognize a particular receptor on the surface of cells and bind to it.
Here the targeted receptor is called VISTA. By binding to it, W0180 should stimulate your own immune system to increase your own capacity to fight against cancer cells. This potential anti-tumor capacity could be improved when combined with other known therapeutic antibodies especially other immune treatment like pembrolizumab.
Pembrolizumab is an existing immune treatment against cancer. Pembrolizumab is also a therapeutic antibody.
Participants receiving W0180 alone will have one administration per week.
Participants receiving W0180 in combination with pembrolizumab will have one administration of W0180 per week and one administration of pembrolizumab every 3 weeks. Both treatments will be started on the same day.
All treatments are administered by intravenous route.
Participants may receive the treatment as long as they benefit from it meaning that the tumor does not worsen and the treatment is well tolerated, with a limitation of 35 administrations maximum for pembrolizumab
