A Trial of a new drug to Treat Patients With Advanced or Metastatic Solid Cancer Tumors (Ulysse)
A Trial of a new drug to Treat Patients With Advanced or Metastatic Solid Cancer Tumors (Ulysse)
Phase I/II open label dose escalation and dose expansion study of intravenous infusion of W0101, an antibody-drug conjugate, in patients with advanced or metastatic solid tumors. International, multicenter, open label study.
How is the study conducted?
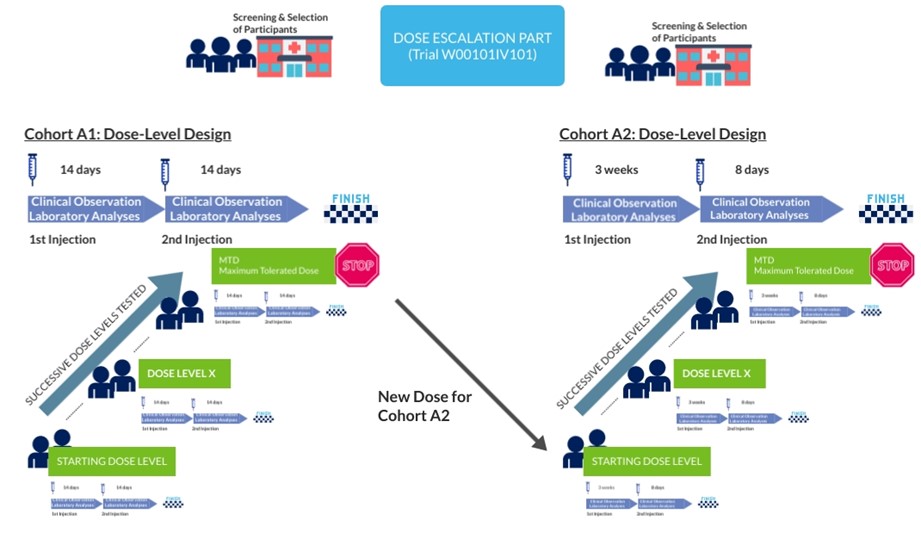
In Phase I, 2 dosing schedules are planned to be tested successively in 2 groups of participants (called cohorts):
- 20 participants will be included in the first cohort (A1)
- 14 participants will be included in the second cohort (A2)
Second part of the trial (Phase II) will be designed when Phase I results will be available.
The schema summarizes the information presented above:
Who can take part in the trial ?
To be part of the trial, participant must fulfill several conditions including the following:
• Adult participants with confirmed advanced or metastatic solid cancer tumors. According to cohort A1 and A2, participants with some specific types of cancer are preferentially selected.
What are the objectives of the trial and how are they evaluated ?
The main objective of the trial is to determine the Maximum Tolerated Dose (MTD) that is to say the highest dose of W0101 that can be administered without exposing participant to an unacceptable health risk.
The MTD is determined by testing increasing doses on different groups of participants (dose level) until the highest dose with acceptable side effects is found.
In addition, the trial will allow :
- To describe the safety of W0101 by evaluating the number and type of side effects
- To describe how the treatment is taken into, move around and eliminated from the body
- To provide preliminary data on the activity of W0101 against cancer tumors
What is the study Medication ?
W0101 (lonigutamab ugodotin), is an Antibody Drug Conjugate (ADC).
An ADC is composed of two main parts: an antibody and a cancer- fighting agent (cytotoxic agent) attached to it. An antibody is a molecule pertaining to the immune system that can specifically recognize a particular receptor expressed on the surface of tumour cell (here IGF-1R) and bind to it. Once bound, the cancer fighting agent has the capacity to kill the cancer cell effectively while sparing the body's healthy cells and thus limit the adverse effects of chemotherapy.
This treatment will be administered by intravenous route (infusion) in 2 different administration schedules:
- Cohort A1: administered every 2 weeks
- Cohort A2: administered every 3 weeks
Participants may receive W0101 as long as they benefit from it that means as long as the tumors do not worsen and the W0101 is well tolerated.
Results
Study primary results :
This is a summary of the main results and conclusions of the trial. Please note that:
- These are the results from all the participants combined. The individual results of each participant might be different and are not in this summary.
This summary reflects the outcome of one single trial and that other trials may show other results or other outcomes.
