A CLINICAL TRIAL TO LEARN ABOUT THE SAFETY AND EFFECTS OF STX-241, A NEW ORAL ANTICANCER DRUG FOR THE TREATMENT OF PATIENTS WITH ADVANCED LUNG CANCER- (STX-241 FIH STUDY)
A CLINICAL TRIAL TO LEARN ABOUT THE SAFETY AND EFFECTS OF STX-241, A NEW ORAL ANTICANCER DRUG FOR THE TREATMENT OF PATIENTS WITH ADVANCED LUNG CANCER- (STX-241 FIH STUDY)
Study of FIH of STX-241 in locally advanced or metastatic NSCLC resistant to EGFR TKIs (STX-241FIH)
How is the study conducted?
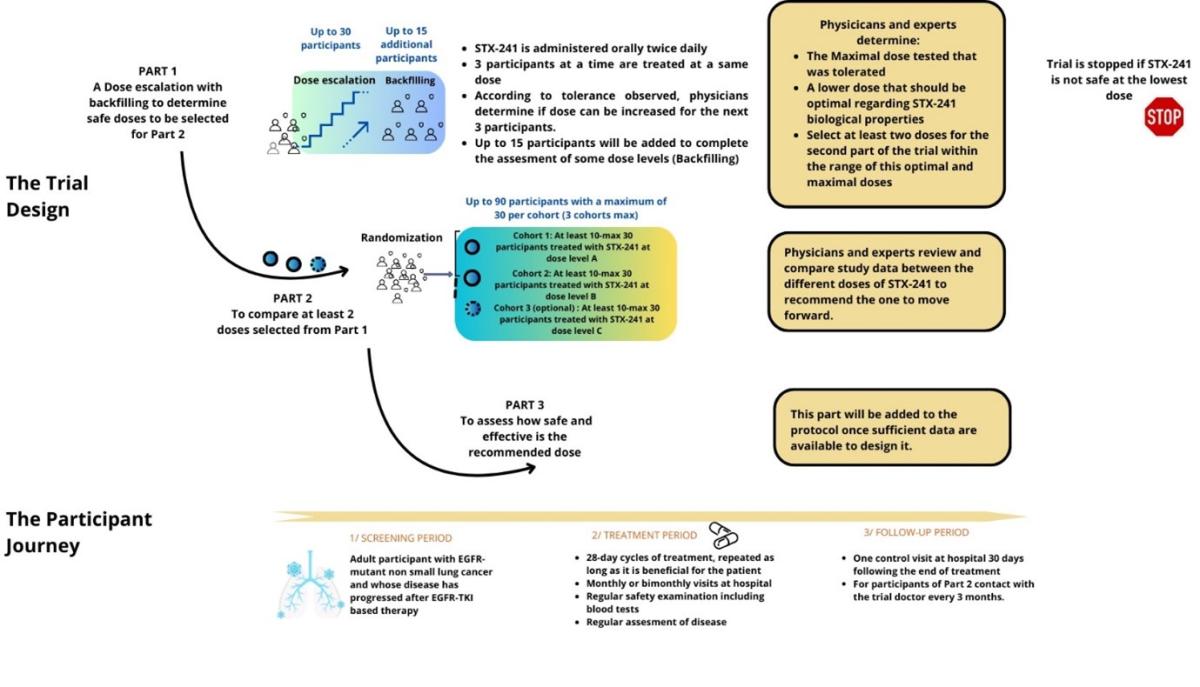
The clinical trial has 3 successive and separate parts:
- The first part (Part 1 - Dose Escalation and Backfilling components) seeks to establish how safe and well tolerated STX-241 is, to determine the highest tolerated dose of STX-241, to identify at least two safe doses with preliminary activity of STX-241 that will be tested in Part 2 of the study and to find out more about its potential side effects.
For the Dose Escalation component, increasing doses of STX-241 will be administered to successive groups of participants until reaching the highest dose that can be given with acceptable side effects. After each dose level, a committee formed of trial doctors (investigators) and Sponsor’s representatives will review the data and decide whether to proceed to the next dose level depending on STX-241 tolerability. Up to 30 participants, who have lung cancer will participate in Part 1 Dose Escalation component.
For the Backfilling component, additional participants who have lung cancer with C797X mutation will enter at doses that have been assessed as safe during the Dose Escalation component. After each dose level, the same committee formed of trial doctors and Sponsor’s representatives will review the data and decide whether additional participants with the C797X mutation will enter in this Backfilling component. Up to 15 participants will participate in Part 1 Backfilling component.
- The second part (Part 2 – Dose Optimization) will determine the best dose of STX-241 between at least two doses identified in Part 1. It will further evaluate the side effects of STX-241 and will explore if STX-241 has some effect on lung cancer.
- The third part (Part 3 – Expansion part) will be added to the protocol once sufficient data are available to design this part.
In all parts of the clinical trial STX-241 will be given orally, two times daily until participant disease progresses or there are side effects requiring stopping treatment with STX-241. Visits and assessments will be organized in cycles of 28 days.
Who can take part in the trial ?
Approximately 135 participants will enter the clinical trial around the world: up to 45 in Part 1 (with up to 30 participants in the Dose Escalation and up to 15 in the Backfilling component), and up to 90 in Part 2 (with at least 10 and up to 30 participants per dose level).
To participate, patients must be at least 18 years old and must have non-small cell lung cancer (with the presence of C797X and the absence of T790M mutations, this being applicable for Backfilling component of Part 1 and Part 2 only) and the last treatment received that was not successful in controlling the growth of their tumour.
In addition, patients must be able to perform their daily activities without much help and should have normal or near to normal blood tests for liver, kidney, and bone marrow functions. If patients are of childbearing potential or patients partner is of childbearing potential, patients need to understand the requirements for contraception, listed later in this information sheet. If patients are women of childbearing potential, they must not be pregnant or breast feeding. Patient must be willing and able to sign an Informed Consent Form.
The trial doctors will need to judge if patients are healthy enough to qualify for the clinical trial and if their participation is medically appropriate.
What are the objectives of the trial and how are they evaluated ?
Primary objectives : How are they evaluated?
Part 1: To characterize the safety and tolerability of STX-241.
(To make sure it's safe for people to use and to see if they can handle any side effects.)
Assess any side effects associated with the drug STX-241, including changes in physical examination results, vital signs, laboratory values and heart function until 30 days after the end of the treatment to determine how well participants can receive treatment with STX-241 without experiencing severe or unacceptable side effects.
Part 1: To determine the range of doses for Part 2, the optimal biologically active dose (OBD) and the maximum tolerated dose (MTD), of STX-241.
(To identify at least two safe doses of STX-241 between the maximum and the minimal effective dose.)
To find the correct dose for the treatment, STX 241 will be given to successive small groups of participants, increasing the dose between groups. To increase the dose safely between groups the researchers need to know how many participants in each dose group will have Dose-limiting toxicities (DLT) during their first 28-days of treatment (a treatment cycle), and what the DLTs are. DLTs are certain types of toxicities caused by taking STX-241 which indicate that the current dose and higher doses may not be tolerated. When DLTs are observed depending on their number and nature, no higher dose may be tested, and lower doses may be considered for further studies of STX-241.
The researchers need to define the Optimal Biological Dose (OBD) and the Maximum Tolerated Dose (MTD). The OBD will be determined by evaluating the occurrence of DLTs during the first cycle of treatment, as well as examining the drug's distribution in the body, tumor shrinkage and molecular signs of drug efficacy (ctDNA reduction) and any moderate or severe adverse events. The MTD will be determined by assessing the occurrence of DLTs during the first cycle of treatment.
Part 2: To determine the Recommended Phase II dose of STX-241
(To assess which one of the doses identified in Part 1 might be the most adequate for Part 3.) For the selected doses (at least two) for Part 2 assess:
- how STX-241 enters the body, circulates in the blood stream and is finally eliminated.
- how well STX-241 works in shrinking or controlling the tumor (response to treatment).
- how safe STX-241 doses are
To further characterize the overall safety of STX-241
- Further evaluate the number and types of side effects (See Part 1) and tolerability whether dose where interrupted, reduced or stopped
All parts: this clinical trial will also evaluate:
- How long STX-241 will allow participant to live without the disease getting worse (Progression free survival: PFS)
- The period of time from when a participant’s tumor shrinks to when it grows again (Duration of response: DOR)
- The percentage of participants whose disease has either shrunk, stayed the same, or not progressed over a certain period of time.
- The period of time from the start of treatment until participants’ tumor shrinks (time to response: TTR)
- The period of time that participants continue to live after their initial diagnosis or treatment, regardless of whether the disease gets better or worse. (Overall survival: OS)
What is the study Medication ?
What is the study Medication ?
PFL-241/STX-241 is a molecule that may work specifically against C797X mutations, which are most frequently found in cancer cells of patients with lung cancer that is no longer responding to a specific type of therapy called a third generation EGFR-inhibitor.
STX-241 treatment should be taken orally in the form of tablets twice a day (ideally in the morning and evening), under fasted condition (1 hour before or at least 2 hours after eating any food).
STX-241 tablets will be administered on a 4-weeks cycle (28-day cycle).
