An observational study to describe treatment patterns of patients with BRAFV600E mutant metastatic non-small cell lung cancer (OCTOPUS).
An observational study to describe treatment patterns of patients with BRAFV600E mutant metastatic non-small cell lung cancer (OCTOPUS).
An observational study to describe treatment patterns of patients with BRAFV600E mutant metastatic non-small cell lung cancer - OCTOPUS.
How is the study conducted?
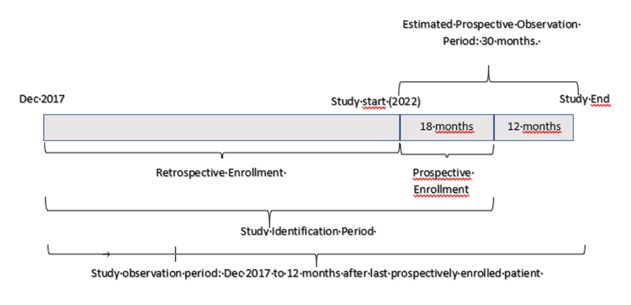
The Study Identification Period is the time period eligibility to select patients who initiated treatment from December 2017 until approximately 200 patients have been enrolled.
The schema depicted below summarizes the study:
Who can take part in the trial ?
To be part of the study, participant must fulfill several conditions including the following:
• Must be aged 18 or older at the time of first-line treatment initiation for mNSCLC.
• Must have initiated a first systemic treatment line for the mNSCLC from 1st December 2017.
• Must have signed Informed Consent Form or non-opposition to study participation.
What are the objectives of the trial and how are they evaluated ?
The primary objective of the study is to describe treatments patterns for first- and second- systemic treatments lines given to adult patients with BRAFV600E mNSCLC.
The secondary objectives of OCTOPUS are to:
• Describe treatment patterns for subsequent treatment lines (beyond first- and second-line).
• Describe demographic and clinical characteristics by collecting data such as age, gender, comorbidities, date of initial diagnosis…
• Describe efficacy of treatments and assess the evolution of patients’ quality of life through questionnaires.
• Describe side effects related to first- and second-line treatments.
