A trial to evaluate whether the combination encorafenib and binimetinib is safe and effective in Chinese participants with BRAFV600E lung cancer
A trial to evaluate whether the combination encorafenib and binimetinib is safe and effective in Chinese participants with BRAFV600E lung cancer

Multicenter, Open-label, Phase II Study with a Safety Lead-in part Investigating the Efficacy, Safety and Pharmacokinetics of Encorafenib and Binimetinib Combination in BRAFV600E mutated Chinese Patients with Metastatic Non-Small Cell Lung Cancer who are BRAF- and MEK inhibitor treatment-naïve
How is the study conducted?
A minimum of fifty-five (55) adult Chinese participants with BRAFV600E metastatic non-small cell lung cancer are in this open-label trial.
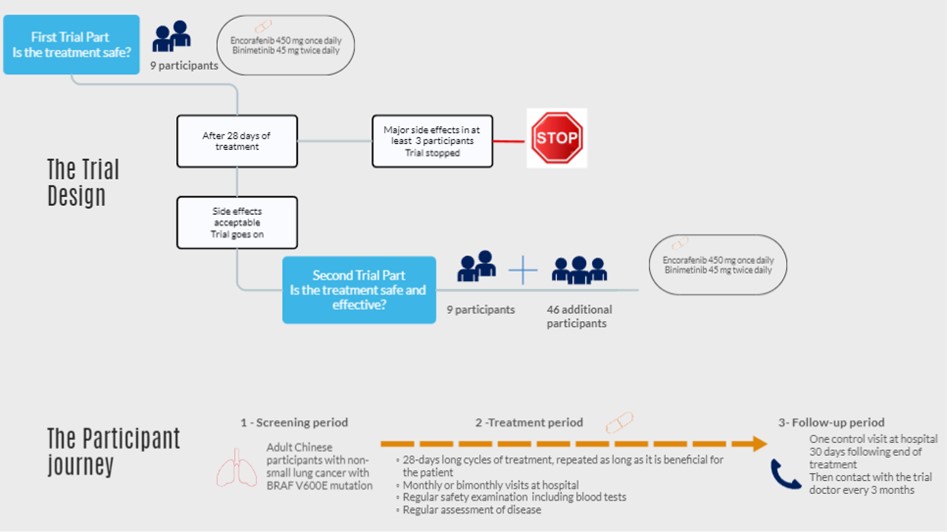
First part of the trial:
Nine (9) participants are in this first part and are treated with encorafenib and binimetinib. After 28 days (one cycle) of treatment for the first nine participants, an analysis is performed to evaluate the safety of encorafenib and binimetinib.
If at least three (3) participants have a major side effect, encorafenib and binimetinib are considered not safe and the trial is stopped.
If the treatment is sufficiently safe, the second part of the trial starts.
The trial
Forty-six (46) additional participants take part in the trial and are treated with encorafenib and binimetinib.
If side effects are acceptable, all participants are treated with encorafenib and binimetinib as long as they benefit from the treatment. That means until the tumor grows or the cancer spreads (disease progression).
When the participant stops the treatment, the follow-up period begins. This period consists of monitoring the participant's health over time, which may include information on their health status, physical examinations, blood tests and imaging tests.
The schema summarizes the information presented above:
Who can take part in the trial ?
be part of the trial, participant must fulfill several conditions including the following:
• Chinese participants with age ≥ 18 years old for China mainland participants and ≥ 20 years old for Taiwan participants with metastatic non-small cell lung cancer
• The tumor cannot be removed by surgery
• The tumor has a specific genetic mutation called BRAFV600E mutation
• Participants have been treated once for their metastatic cancer or they have never been treated
• Participants have never been treated with the same type of treatment to encorafenib and binimetinib for their metastatic cancer
• Participants are not pregnant, lactating or breast-feeding women
What are the objectives of the trial and how are they evaluated ?
The main objective of the first part of the trial is:
• To investigate the safety (side effects) of encorafenib 450 mg and binimetinib 90 mg in Chinese participants with BRAFV600E metastatic non-small cell lung cancer. This is measured with the number of participants with major unacceptable side effects during the first 28 days of treatment.
The main objective of the second part of the trial is:
• To see if the combination of encorafenib and binimetinib is effective in this type of lung cancer by counting the proportion of participants for whom the tumors shrink or completely disappear after treatment (overall response rate).
In addition, the trial allows:
• For the first and second parts, to describe the safety (side effects) of encorafenib and binimetinib and how the treatment enters the body, circulates in the bloodstream and is finally eliminated with safety data including the number, frequency and type of side effects.
• For the second part, to further investigate the efficacy of the combination of encorafenib and binimetinib:
− The time period the response to treatment is maintained (duration of response).
− The time period between the treatment starts and the tumor becomes worse (progression-free survival).
− The time period since the treatment start and participant's death (overall survival).
What is the study Medication ?
Participants treated with the new therapy receive:
• 6 capsules of encorafenib once a day in the morning. The total daily dose of encorafenib is 450 mg
• 3 tablets of binimetinib twice a day (in the morning and in the evening). The total daily dose of binimetinib is 90 mg
