A trial to evaluate whether encorafenib is safe in Chinese mainland participants with BRAFV600E advanced solid tumors
A trial to evaluate whether encorafenib is safe in Chinese mainland participants with BRAFV600E advanced solid tumors

A multicenter, open-label, phase 1 study investigating the safety and tolerability of encorafenib monotherapy in BRAFV600E-mutated Chinese patients with advanced metastatic solid tumors
How is the study conducted?
This trial is an open-label “Phase 1” trial in Chinese mainland population.
A maximum of 6 adult Chinese participants (18 years and older) are in this trial.
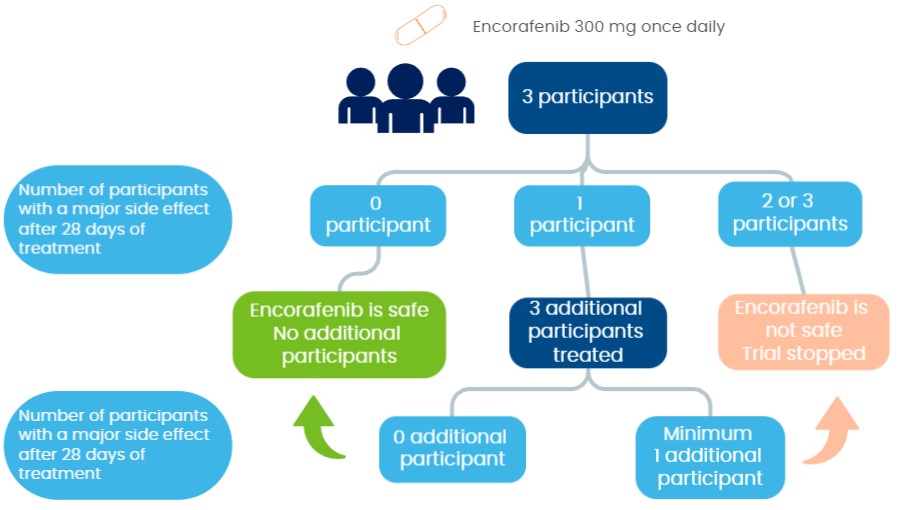
Three participants first take part in the trial and are treated with encorafenib. After 28 days (one cycle) of treatment for the first three participants, an analysis is performed to investigate the safety of encorafenib:
-If no participant has a major unacceptable side effect, no further participants take part in the trial.
-If one participant has a major unacceptable side effect, three (3) additional participants are treated with encorafenib to investigate the safety of the treatment.
-If two (2) or three (3) participants have a major unacceptable side effect, encorafenib is not safe and the trial is stopped.
Encorafenib is considered sufficiently safe if there is no more than 1 participant with observed major unacceptable side effects .
If sufficiently safe, all participants are treated with encorafenib as long as they benefit from the treatment. That means until the tumor grows or the cancer spreads (disease progression).
The schema summarizes the information presented above:
Who can take part in the trial ?
To be part of the trial, participant must fulfill several conditions including the following:
• Chinese mainland participants, aged 18 years or older with skin cancer (melanoma) or lung cancer (non-small cell lung cancer) and whose cancer has spread to other places of the body (metastatic).
• The tumor has a specific genetic mutation called BRAFV600E mutation
• Participants have been treated for their metastatic cancer but the previous treatment didn't work
• Participants have never been treated for their metastatic disease with a treatment targeting the BRAF mutation
• Participants are not pregnant, lactating or breast-feeding women
What are the objectives of the trial and how are they evaluated ?
The main objective of the trial is:
• To investigate the safety (side effects) of encorafenib 300 mg during the first 28 days of treatment (first cycle). This is assessed by measuring the number of Chinese participants with major unacceptable side effects during the first 28 days of treatment (first cycle).
In addition, the trial allows :
• To describe the safety of encorafenib 300 mg during the total treatment period and how the treatment enters the body, circulates in the bloodstream and is finally eliminated, evaluating the number, frequency and type of side effects that occurred in participants until 30 days after the last dose of treatment.
What is the study Medication ?
Participants receive 4 capsules of encorafenib once a day in the morning. The total daily dose of encorafenib is 300 mg.
Results
Study primary results :
This is a summary of the main results and conclusions of the trial. Please note that:
- These are the results from all the participants combined. The individual results of each participant might be different and are not in this summary.
This summary reflects the outcome of one single trial and that other trials may show other results or other outcomes.
