A trial to evaluate whether encorafenib in combination with cetuximab is safe and effective in the Chinese mainland population with BRAFV600E mutant metastatic Colorectal Cancer.
A trial to evaluate whether encorafenib in combination with cetuximab is safe and effective in the Chinese mainland population with BRAFV600E mutant metastatic Colorectal Cancer.

A multicenter, randomized, open-label, 2-arm, Phase II trial with a safety lead-in phase evaluating the combination of encorafenib and cetuximab versus irinotecan/cetuximab or infusional 5-fluorouracil (5-FU)/folinic acid (FA)/irinotecan (FOLFIRI)/cetuximab in Chinese patients with BRAFV600E mutant metastatic colorectal cancer.
How is the study conducted?
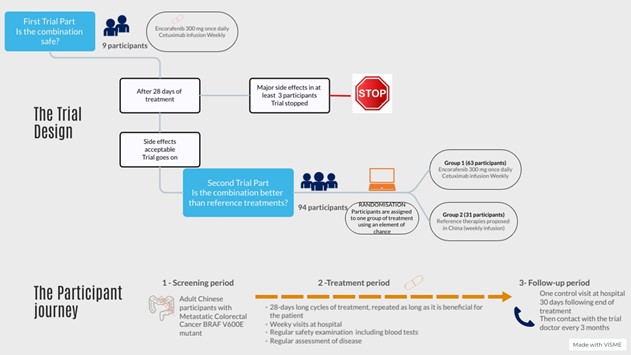
This is a Phase 2 trial with 103 participants.
During the first part of the trial, nine (9) participants are treated with the combination of encorafenib and cetuximab.
After 28 days (one cycle) of treatment, an analysis is performed to check whether the combination is safe.
If at least three (3) participants have a major side effect, the combination is considered not safe, and the trial is stopped.
If the combination is considered safe with less than three (3) participants having a major side effect, the trial proceeds to the second part.
Ninety-four (94) additional participants enter the second part of the trial and are splitted in two groups using an element of chance (randomization):
• Two-thirds of participants (63) receive the combination
• One-third of participants (31) receive the reference treatments
Both parts of the trial start with a screening phase to assess wether the participants fulfills all the conditions to enter the trial.
The treatment period starts with the first dose received and consists in:
• Successive cycles of treatments, 28 days long each during which therapies are administrered according to the trial protocol
• Regular assessments to control participant's safety including physical examinations and blood tests
• Regular assessments including imaging tests to control the size of the tumors and the evolution of the disease.
All participants are treated as long as they benefit from the treatment received. That means as long as the tumors do not worsen (disease progression) and the treatment is tolerated.
When the participant stops the treatment, the follow-up period begins. This period consists of monitoring the participant's health over time, which may include information on their health status, physical examinations, blood tests and radiological imaging.
The schema summarizes the information presented above:
Who can take part in the trial ?
To be part of the trial, participant must fulfill several conditions including the following:
The participant is Mainland Chinese with age ≥ 18 years old
The tumor cannot be removed by surgery (unresectable)
The tumor has a specific genetic mutation called BRAFV600E mutation
The participant has been treated once or twice for his/her metastatic cancer before
The participant has never been treated with the treatments studied in the trial
What are the objectives of the trial and how are they evaluated ?
There are 2 main objectives assessed in two consecutive parts of the trial:
• The purpose of the first part is to evaluate whether the combination of encorafenib and cetuximab is safe by primarily counting the number of participants who had major side effect during the first 28 days of treatment.
• The purpose of the second part is to compare the combination with the reference treatments. Efficacy is primarily assessed by analysing the period of time between the start of treatment and the disease worsens
In addition, thanks to the trial, researcher intend to :
• Describe the side effects in Chinese population and compare the combination with the reference treatments in term of tolerance.
• Describe how encorafenib enters the body, circulates in the bloodstream and is finally eliminated.
• Compare the combination with the reference treatments in term of survival that is to say the period of time between treatment assignement and the participant's death (overall survival)
• Compare the combination with the reference treatments in term of response rate that is to say the proportion of participants for whom the tumors shrink or completely disappear after treatment.
What is the study Medication ?
Participants treated with the combination receive :
• 4 capsules of encorafenib to be taken once a day in the morning. The total daily dose of encorafenib is 300mg.
• Cetuximab by intravenous infusion performed once a week at the hospital.
Participants treated with the reference treatments receive either irinotecan and cetuximab or FOLFIRI and cetuximab at the investigator’s discretion. All these treatments are administered by intravenous infusion performed at the hospital (once a week for Cetuximab and every two weeks for irinotecan and FOLFIRI).
