A trial to evaluate whether encorafenib in combination with binimetinib is effective and safe in participants with high risk Stage II BRAF-mutated melanoma
A trial to evaluate whether encorafenib in combination with binimetinib is effective and safe in participants with high risk Stage II BRAF-mutated melanoma

Adjuvant encorafenib & binimetinib vs. placebo in resected stage II BRAF V600E/K mutated melanoma: a randomized triple-blind Phase III Study in collaboration with the EORTC Melanoma Group.
How is the study conducted?
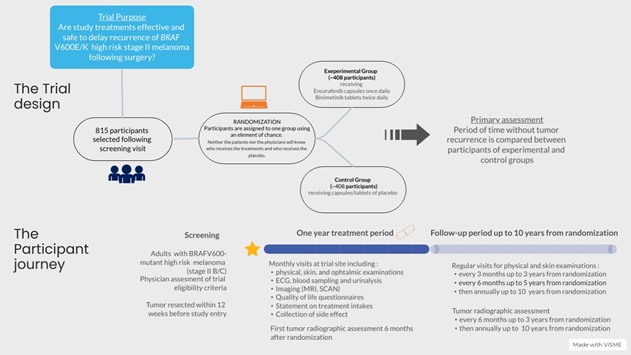
This trial is a triple blind randomized Phase III trial. Approximately 815 participants aged 18 years and more, will join the study around the world.
Through the randomization process, ie. using an element of chance, participant will be assigned to a study group: receiving either the encorafenib and binimetinib (experimental group) or their respective placebos (control group). There is a one in two chance of getting the encorafenib and binimetinib, and a one in two chance of getting their placebos.
The study treatments (encorafenib and binimetinib combination or their placebos) will be administrated for a maximum duration of 12 months. During this period, there will be regular monthly visits to the study site.
After the treatment period, Participants will have to come back to the study site for a safety follow-up visit, approximately 30 days after the last dose of study treatment and to continue to perform clinical and imaging exams to follow the evolution of their melanoma for up to 10 years.
The scheme summarizes the information presented above:
Who can take part in the trial ?
To be part of the trial, participant must fulfill several conditions including the following:
• Be aged 18 years or older with high risk melanoma (Stage IIB/C)
• Had recent (<12 weeks) surgery to completely remove the melanoma (resection)
• The tumor has a specific genetic mutation (BRAF V600E/K) called BRAF mutation
• Do not be pregnant, lactating or breast-feeding women
What are the objectives of the trial and how are they evaluated ?
The main objective will be to evaluate whether treatment with encorafenib and binimetinib prolongs the period of time without recurrence after melanoma has been removed by surgery (recurrence-free survival RFS) as compared to placebo in patients with stage IIB/C BRAF V600E/K melanoma.
In addition, the trial will allow to:
-Assess whether the treatment with encorafenib and binimetinib prolongs the period of time without the skin tumor could spread in the body as compared to placebo (distant metastasis free survival(DMFS)) and prolongs the period of time between treatment assignment and the participant death (overall survival (OS))
-Assess the participant quality of life during the treatment
-Assess the safety and tolerability of the treatment by collecting the frequency and type of side effects that occurred in participants all along the trial and the degree to which overt side effects will be tolerated by the participant
What is the study Medication ?
Encorafenib (BRAFTOVI®) Dose: 450 mg (6 x 75 mg); Frequency: Once a day; Route of administration: oral (capsule)
Binimetinib (MEKTOVI®) Dose: 45mg (3x15mg); Frequency: Twice a day; Route of administration: oral (tablet)
For participants randomized to the control arm, they will receive encorafenib and binimetinib placebos
Duration of administration: 12 months but treatment could be stopped before if melanoma recurs (re-appears at the initial disease site, a nearby area or an area distant from the original site) or if the treatment is not tolerated.
More information
This trial is conducted in collaboration with the European Organisation for Research and Treatment of Cancer – EORTC
EORTC is an independent, non-governmental, non-profit cancer research Organisation established under the laws of Belgium, its mission is to coordinate and conduct international translational and clinical research to improve the standard of cancer treatment for patients. In addition to independence, EORTC is recognised for scientific and methodological rigor bringing robust datasets to doctors and patients for therapeutic improvement. EORTC covers all disciplines to fight against cancer. EORTC research leaves no one behind and addresses all patients, including patients with rare tumours and specific patient populations.
