A trial to demonstrate that administration of binimetinib treatment using a 45 mg strength tablet is equivalent to 3 tablets of 15 mg.
A trial to demonstrate that administration of binimetinib treatment using a 45 mg strength tablet is equivalent to 3 tablets of 15 mg.
A randomized, single-center, open-label, single dose, two-period, crossover pivotal bioequivalence study comparing binimetinib 3 x 15 mg and 45 mg tablets in healthy participants
How is the study conducted?
This is a randomized, crossover Phase I trial.
Randomized means that healthy volunteers will be split in two groups using an element of chance:
• one group will receive the reference formulation first (3 tablets of 15 mg) then the test formulation (one tablet of 45 mg)
• one group will receive the test formulation first then the reference formulation.
Crossover means that each healthy volunteer will sequentially receive both formulations.
The trial will consist of:
• A screening period before the first treatment administration to check that participant fulfills all conditions to enter the trial
• A first treatment period of 5 days, requiring overnight stay at trial center for the first 3 days following the first administration.
• A washout period of at least 7 days required for the body to eliminate drug from first administration.
• A second treatment period of 5 days, requiring overnight stay at trial center for the first 3 days following the second administration.
• An End-of-Study visit to be performed 1 month after last administration for a final examination of the participant.
The trial will be carried out in a center specialized in phase 1 trials with experienced medical staff and adequate facilities.
The schema summarizes the information presented above:
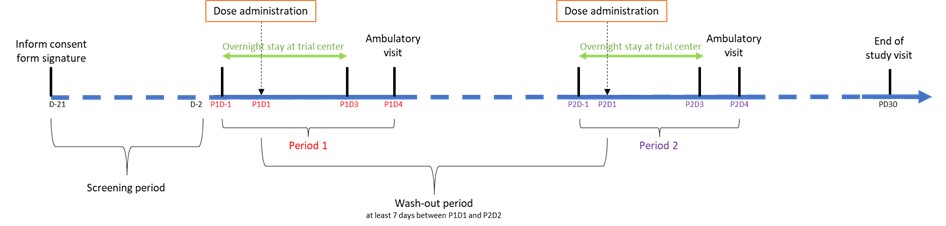
P1D1 stands for "Period 1 Day 1"and corresponds to the day of administration during this first period
P2D1 stands for "Period 2 Day 1"and corresponds to the day of administration during this second period
Who can take part in the trial ?
To be part of the trial, participant must fulfill several conditions including the following:
• Healthy volunteer as assessed by clinical examinations during screening period (including blood test) and medical history
• Aged between ≥ 18 and ≤ 65 years, except pregnant women and women of childbearing potential
What are the objectives of the trial and how are they evaluated ?
The primary objective of the trial is to compare the concentration of binimetinib in the blood after administration of both formulations.
Measure of concentration at different time points following administration provides estimation for :
• the total exposure to binimetinib experienced by the participant. The total exposure is the amount of treatment circulating in the blood from administration to elimination.
• the maximal concentration observed in the blood
These are primary pharmacokinetics parameters used to assess bioequivalence.
In addition, the trial will allow:
• To compare additional pharmacokinectic parameters such as the time between treatment administration and observation of the maximal concentration in the blood
• To evaluate the safety of both formulations of binimetinib according to the number and type of side effects
What is the study Medication ?
Participants will receive 2 single oral administrations of 45 mg binimetinib, separated by a period of at least 7 days between intakes.
• One administration will be performed using 1 tablet of 45 mg (the test formulation)
• One administration will be performed using 3 tablets of 15 mg (the reference formulation currently marketed)
Results
Study primary results :
This is a summary of the main results and conclusions of the trial. Please note that:
- These are the results from all the participants combined. The individual results of each participant might be different and are not in this summary.
This summary reflects the outcome of one single trial and that other trials may show other results or other outcomes.
