
Why Participation Matters?
Clinical trials are indispensable to evaluate the effects of a treatment, to determine if it's safe and effective and should be made available to the public.
Participating in clinical trials means making possible the discovery and development of treatments that might improve or cure a disease. These might be new drugs or combinations, new surgical procedures or devices, or new ways to use existing treatments. The progress in medicine depends on the results of clinical research and patient centric clinical trials conducted with rigor and method, guaranteeing a good level of scientific proof.
As healthy volunteers, participants contribute to moving science forward, not only for a specific disease but also for other scientific fields. Participants with a disease, have the opportunity to receive innovative treatments not available in the usual care and access to experienced medical teams that carefully monitor their disease and overall health. Future patients may have the opportunity to benefit from a certain therapy tested in clinical trials and have their disease improved or cured.
Why do we need a certain numbers of participants ?
As each human being is unique and may react in a different way to a drug, as the efficacy of the drug may vary from one individual to another and some participants will experience side effects, some will not:
Individual information is crucial for clinical research but isolated, neither sufficient to provide evidence for drug efficacy nor for full description of safety.
This requires aggregation of participant experiences within a trial and combining multiple trials.
More Information:
The number of participants to be enrolled is set in the protocol and directly related to the primary question raised. Thanks to clinical hypothesis and statistical methods, scientists determine the appropriate number of participants that are needed to answer this question with a sufficient level of confidence.
How is a Participant protected ?
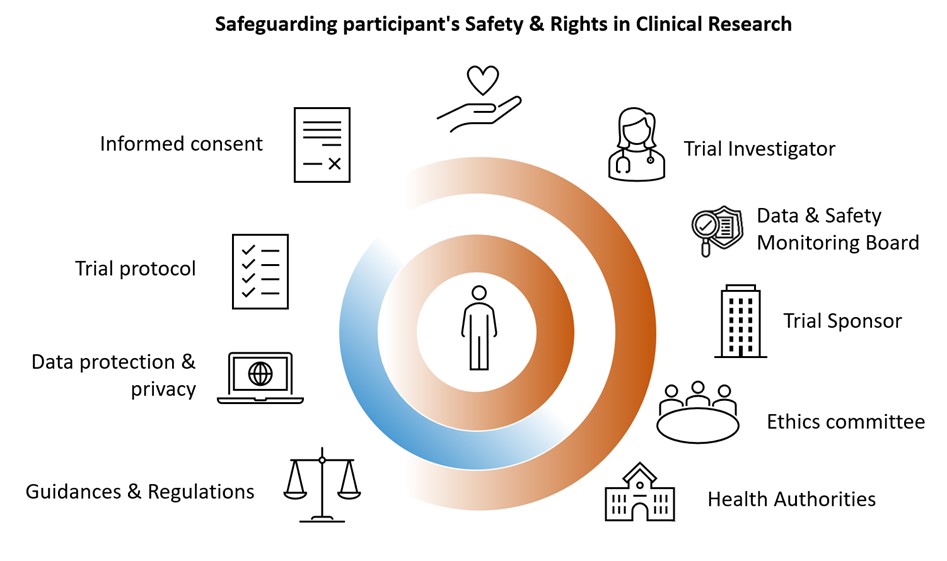
The protection of participants, a primary concern of clinical research, is governed by founding text and guidelines.
Clinical research is conducted with human and ethical principles that govern medical practice. It must comply with standards, applicable local and international regulatory requirements that ensure adequate protection of clinical trial participants. Clinical trial regulations, laws and guidelines aim to protect and guarantee the rights, safety and well-being for all participants (minors, protected adults, adults, sick or vulnerable persons, healthy volunteers), as well as their data and biological samples (blood, tissue, organs). The interest of the participants must always take precedence over those of science and society. In addition, many entities provide oversight of clinical trials throughout their duration, such as Health Authorities, Ethics Review Boards, Data and Safety Monitoring Boards.
Participant protection also includes preserving privacy (5,6), keeping information confidential, allowing the participant to remain anonymous, providing adequate information about the trial with the informed consent process, giving the participant the opportunity to ask questions, and allowing the participant to stop participating in the trial at any time.
Hyperlinks to text and guidelines:
- ICH Good Clinical Practices: ICH GCP
- Helsinki Declaration: Declaration of Helsinki
- European directives: 2001/20/EC and 2005/28/EC
- European regulation: Regulation 536/2014
- General Data Protection Regulation: GDPR
- FDA: Code of Federal Regulations
